Background:
Warfarin has been shown in pre-clinical models to have anti-neoplastic activity. In a population-based study using the SEER-Medicare Linked Database warfarin was associated with improved survival compared to low molecular weight heparin (LMWH) or direct oral anticoagulants (DOACs) in the treatment of cancer-associated thrombosis (CAT). To assess whether the observed survival advantage is consistent in other large cohorts, we performed a matched cohort study utilizing the Veterans Affairs (VA) database. The VA is a large, integrated health care system with comprehensive data for their patients at the individual level, which provides the optimal observational cohort to confirm the novel hypothesis warfarin confers a mortality benefit over LMWH.
Method:
Using the retrospective data from the VA health care system from 2000-2021, we identified patients with CAT who were > 18 years, had available prescription data for anticoagulation within 30 days of venous thromboembolism (VTE), and survived > 14 days after the index VTE event. Patients were excluded if they had prior diagnosis of VTE or had any anticoagulation prescription prior to the first coded VTE event. The primary outcome was overall survival (OS). Patients were matched 1:1 using nearest neighbor matching on the propensity score. The matching variables included cancer site, cancer stage, date of cancer diagnosis, date of VTE diagnosis, age at VTE diagnosis, gender, race, BMI, Elixhauser-based weighted comorbidity score, and rurality of residential status. Cox proportional-hazards regression was performed to estimate hazard ratios (HR) and 95% confidence intervals. Log-rank test was utilized to estimate the p-value. Subgroup analyses were performed in the largest groups of cancer available in the data set including prostate cancer, lung cancer, gastrointestinal cancer (excluding hepatobiliary cancer), and malignant hematologic neoplasms (including myeloid malignancy, lymphoid malignancy, and multiple myeloma). Finally subgroup analysis was also performed on localized cancer and metastatic disease based on AJCC stage grouping.
Result:
There were a total of 20,078 patients that met the inclusion and exclusion criteria without missing data. The warfarin cohort consistent of 8383 patients while non-warfarin cohort comprised 11696 patients. Among the non-warfarin cohort, 54% of the patients were prescribed LMWH exclusively and 38% of the patients were prescribed DOAC exclusively within 30days of the VTE diagnosis. The most common malignancies in the cohort included prostate (29.4%), lung (20.9%), gastrointestinal (15.7%) and hematologic malignancies (10.3%). Of patients with solid tumors, 22% had metastatic cancer. Among the VTE events, 33.3% were acute lower extremity deep vein thrombosis, 48.4% were acute pulmonary embolism, and 18.3% were acute upper extremity deep vein thrombosis. After propensity score matching, 8,383 patients were included in the warfarin arm and 8,383 patients in the non-warfarin arm. The median age at VTE diagnosis of the matched cohort was 69 [IQR 63 - 75] and the majority of the patients (96.7%) were male.
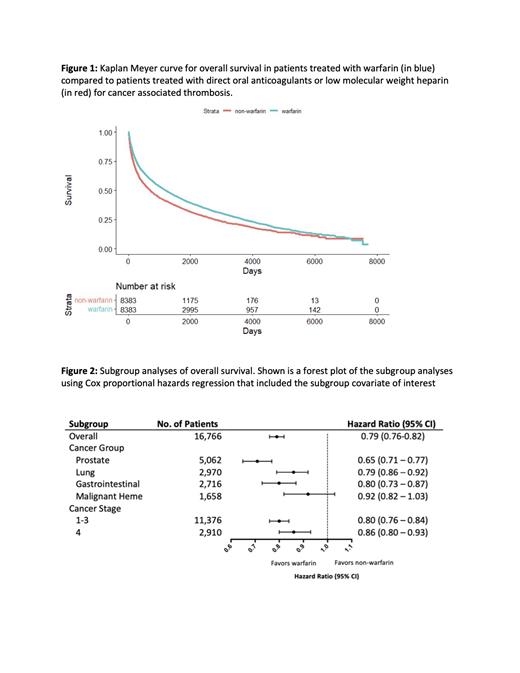
Warfarin was associated with a significant improvement in OS compared to non-vitamin K antagonists (median OS, 1204 days [95% CI, 1137-1276 days] vs 703 days [95% CI, 665-747 days]; HR 0.79 [95% CI 0.76 - 0.82]; p-value <2x10 -16) (Figure1). The difference was also preserved in gastrointestinal malignancy (HR 0.8 v95% CI 0.73-0.87), lung cancer (HR 0.8, 95% CI 0.79-0.92) and prostate cancer (HR 0.71, 95% CI 0.65-0.77). The survival advantage was preserved in patients with metastatic (HR 0.86, 95% CI 0.78-0.83) and localized cancer (HR 0.80, 95% CI 0.76-0.84) (Figure 2).
Conclusion and Relevance:
In a population level propensity matched study, warfarin for the treatment of CAT was associated with significantly improved survival over non-vitamin K antagonist anticoagulation in CAT. These provocative finding confirm the previous observation in SEER-Medicare database and have important implications on patient care and health care costs. Further investigation into the biological basis of these findings could potentially lead to biomarker and drug discovery for cancer therapies and prognostication.
Disclosures
Zwicker:calyx: Consultancy; Sanofi: Consultancy; Janssen: Consultancy; CSL Behring: Consultancy; Sanofi, CSL, Parexel: Consultancy; Incyte Corporation, Quercegen: Research Funding; Pfizer/BMS, Portola, Daiichi: Honoraria.